
Fully automated fast-flow synthesis of antisense phosphorodiamidate morpholino oligomers
Fully automated fast-flow synthesis of antisense phosphorodiamidate morpholino oligomers
Nature Communications volume 12, Article number: 4396 (2021)
Chengxi Li, Alex J. Callahan, Mark D. Simon, Kyle A. Totaro, Alexander J. Mijalis, Kruttika-Suhas Phadke, Genwei Zhang, Nina Hartrampf, Carly K. Schissel, Ming Zhou, Hong Zong, Gunnar J. Hanson, Andrei Loas, Nicola L. B. Pohl, David E. Verhoeven & Bradley L. Pentelute
Abstract
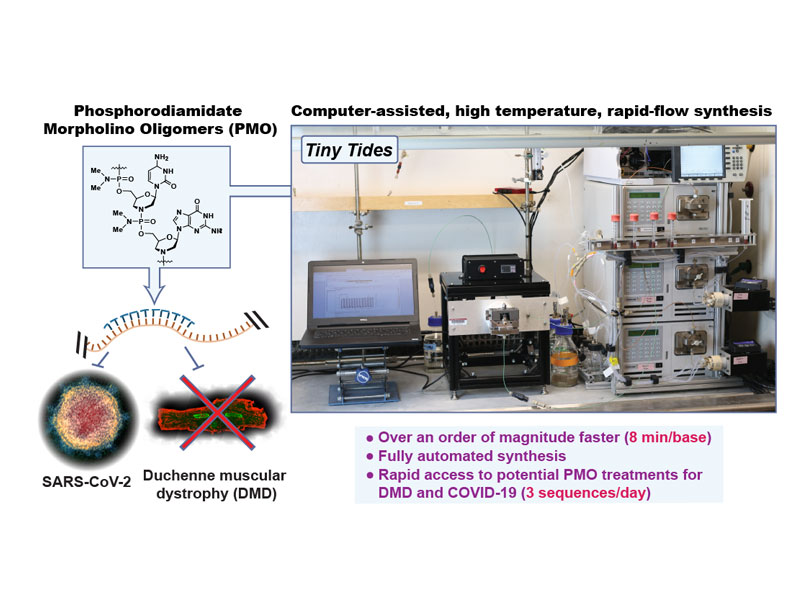
Rapid development of antisense therapies can enable on-demand responses to new viral pathogens and make personalized medicine for genetic diseases practical. Antisense phosphorodiamidate morpholino oligomers (PMOs) are promising candidates to fill such a role, but their challenging synthesis limits their widespread application. To rapidly prototype potential PMO drug candidates, we report a fully automated flow-based oligonucleotide synthesizer. Our optimized synthesis platform reduces coupling times by up to 22-fold compared to previously reported methods. We demonstrate the power of our automated technology with the synthesis of milligram quantities of three candidate therapeutic PMO sequences for an unserved class of Duchenne muscular dystrophy (DMD). To further test our platform, we synthesize a PMO that targets the genomic mRNA of SARS-CoV-2 and demonstrate its antiviral effects. This platform could find broad application not only in designing new SARS-CoV-2 and DMD antisense therapeutics, but also for rapid development of PMO candidates to treat new and emerging diseases.



