
Rapid Chemical Synthesis of Serine Protease Inhibitor Kazal-type 13 (SPINK13) Glycoform by a Combined Method with Glycan Insertion Strategy and Fast-Flow Fmoc SPPS
Rapid Chemical Synthesis of Serine Protease Inhibitor Kazal-type 13 (SPINK13) Glycoform by a Combined Method with Glycan Insertion Strategy and Fast-Flow Fmoc SPPS
Dr. Kota Nomura, Dr. Ryo Okamoto, Dr. Yuta Maki, Ayumu Hayashibara, Prof. Dr. Toshifumi Takao, Dr. Tomoya Fukuoka, Prof. Dr. Eiji Miyoshi, Prof. Dr. Bradley L. Pentelute, Prof. Dr. Yasuhiro Kajihara
Abstract
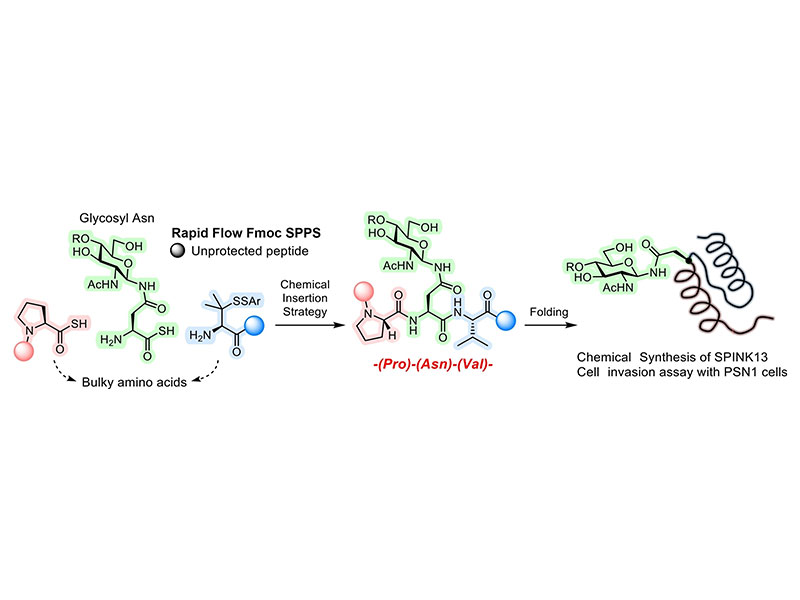
Serine protease inhibitor Kazal type 13 (SPINK13) is a secreted protein that has been recently studied as a therapeutic drug and an interesting biomarker for cancer cells. Although SPINK13 has a consensus sequence (Pro-Asn-Val-Thr) for N-glycosylation, the existence of N-glycosylation and its functions are still unclear. In addition to this, the preparation of glycosylated SPINK 13 has not been examined by both the cell expression method and chemical synthesis. Herein we report the chemical synthesis of the scarce N-glycosylated form of SPINK13 by a rapid synthetic method combined with the chemical glycan insertion strategy and a fast-flow SPPS method. Glycosylated asparagine thioacid was designed to chemoselectively be inserted between two peptide segments where is the sterically bulky Pro-Asn(N-glycan)-Val junction by two coupling reactions which consist of diacyl disulfide coupling (DDC) and thioacid capture ligation (TCL). This insertion strategy successfully afforded the full-length polypeptide of SPINK13 within two steps from glycosylated asparagine thioacid. Because the two peptides used for this synthesis were prepared by a fast-flow SPPS, the total synthetic time of glycoprotein was considerably shortened. This synthetic concept enables us to repetitively synthesize a target glycoprotein easily. Folding experiments afforded well-folded structure confirmed by CD and disulfide bond map. Invasion assays of glycosylated SPINK13 and non-glycosylated SPINK13 with pancreatic cancer cells showed that non-glycosylated SPINK-13 was more potent than that of glycosylated SPINK13.



