
Branched Multimeric Peptides as Affinity Reagents for the Detection of α-Klotho Protein
Branched Multimeric Peptides as Affinity Reagents for the Detection of α-Klotho Protein
Xiyun Ye, Dr. Peiyuan Zhang, John C. K. Wang, Dr. Corey L. Smith, Silvino Sousa, Dr. Andrei Loas, Dr. Dan L. Eaton, Dr. Magdalena Preciado López, Prof. Dr. Bradley L. Pentelute
Abstract
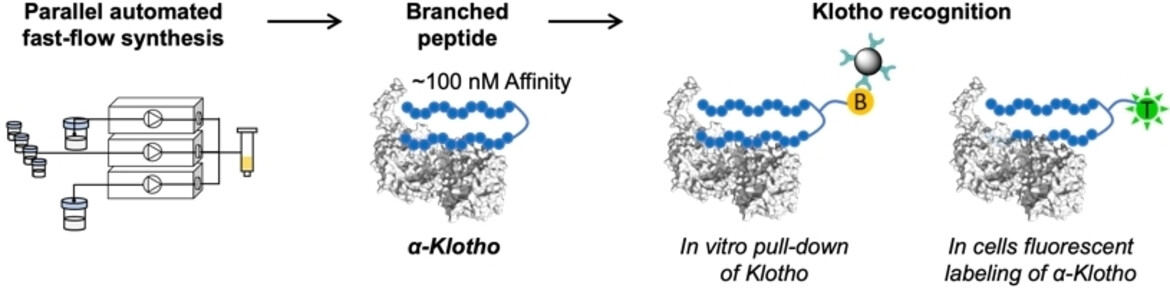
α-Klotho, an aging-related protein found in the kidney, parathyroid gland, and choroid plexus, acts as an essential co-receptor with the fibroblast growth factor 23 receptor complex to regulate serum phosphate and vitamin D levels. Decreased levels of α-Klotho are a hallmark of age-associated diseases. Detecting or labeling α-Klotho in biological milieu has long been a challenge, however, hampering the understanding of its role. Here, we developed branched peptides by single-shot parallel automated fast-flow synthesis that recognize α-Klotho with improved affinity relative to their monomeric versions. These peptides were further shown to selectively label Klotho for live imaging in kidney cells. Our results demonstrate that automated flow technology enables rapid synthesis of complex peptide architectures, showing promise for future detection of α-Klotho in physiological settings.



