
Quantifying residue-specific conformational dynamics of a highly reactive 29-mer peptide
Quantifying residue-specific conformational dynamics of a highly reactive 29-mer peptide
Scientific Reports volume 10, Article number: 2597 (2020
William R. Lindemann, Ethan D. Evans, Alexander J. Mijalis, Olivia M. Saouaf, Bradley L. Pentelute & Julia H. Ortony
Abstract
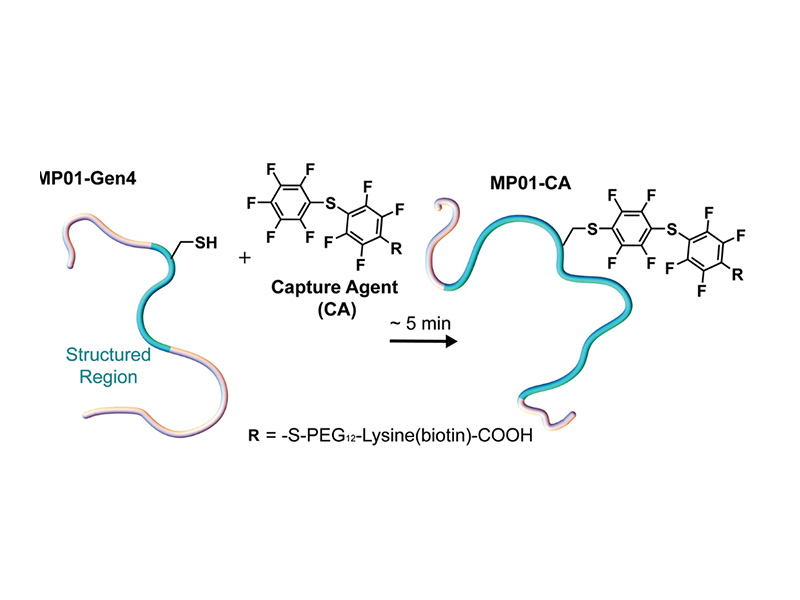
Understanding structural transitions within macromolecules remains an important challenge in biochemistry, with important implications for drug development and medicine. Insight into molecular behavior often requires residue-specific dynamics measurement at micromolar concentrations. We studied MP01-Gen4, a library peptide selected to rapidly undergo bioconjugation, by using electron paramagnetic resonance (EPR) to measure conformational dynamics. We mapped the dynamics of MP01-Gen4 with residue-specificity and identified the regions involved in a structural transformation related to the conjugation reaction. Upon reaction, the conformational dynamics of residues near the termini slow significantly more than central residues, indicating that the reaction induces a structural transition far from the reaction site. Arrhenius analysis demonstrates a nearly threefold decrease in the activation energy of conformational diffusion upon reaction (8.0 kBT to 3.4 kBT), which occurs across the entire peptide, independently of residue position. This novel approach to EPR spectral analysis provides insight into the positional extent of disorder and the nature of the energy landscape of a highly reactive, intrinsically disordered library peptide before and after conjugation.



