
Automated Flow Synthesis of Tumor Neoantigen Peptides for Personalized Immunotherapy
Automated Flow Synthesis of Tumor Neoantigen Peptides for Personalized Immunotherapy
Scientific Reports volume 10, Article number: 723 (2020
Nicholas L. Truex, Rebecca L. Holden, Bin-You Wang, Pu-Guang Chen, Stephanie Hanna, Zhuting Hu, Keerthi Shetty, Oriol Olive, Donna Neuberg, Nir Hacohen, Derin B. Keskin, Patrick A. Ott, Catherine J. Wu & Bradley L. Pentelute
Abstract
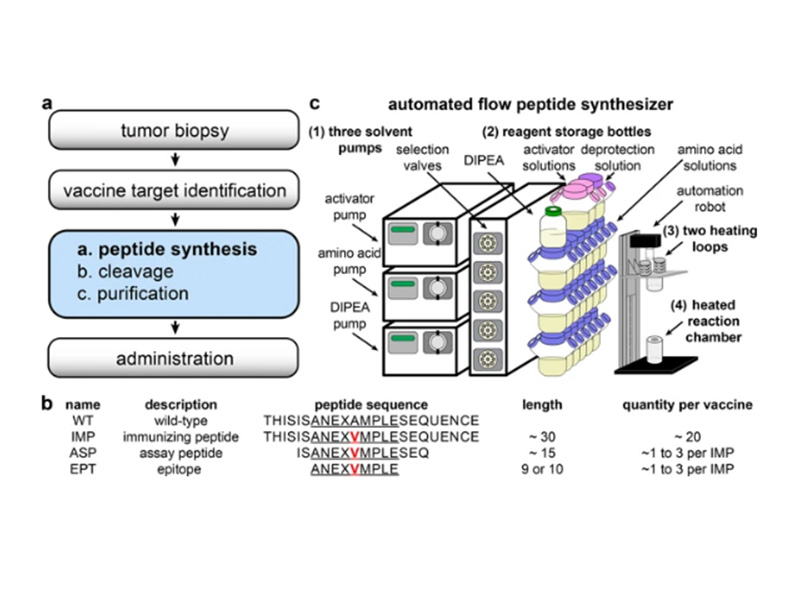
High-throughput genome sequencing and computation have enabled rapid identification of targets for personalized medicine, including cancer vaccines. Synthetic peptides are an established mode of cancer vaccine delivery, but generating the peptides for each patient in a rapid and affordable fashion remains difficult. High-throughput peptide synthesis technology is therefore urgently needed for patient-specific cancer vaccines to succeed in the clinic. Previously, we developed automated flow peptide synthesis technology that greatly accelerates the production of synthetic peptides. Herein, we show that this technology permits the synthesis of high-quality peptides for personalized medicine. Automated flow synthesis produces 30-mer peptides in less than 35 minutes and 15- to 16-mer peptides in less than 20 minutes. The purity of these peptides is comparable with or higher than the purity of peptides produced by other methods. This work illustrates how automated flow synthesis technology can enable customized peptide therapies by accelerating synthesis and increasing purity. We envision that implementing this technology in clinical settings will greatly increase capacity to generate clinical-grade peptides on demand, which is a key step in reaching the full potential of personalized vaccines for the treatment of cancer and other diseases.



