
Nitrogen Arylation for Macrocyclization of Unprotected Peptides
Nitrogen Arylation for Macrocyclization of Unprotected Peptides
J. Am. Chem. Soc., 2016, 138 (27), pp 8340–8343
DOI: 10.1021/jacs.6b03757
Publication Date (Web): June 22, 2016
Guillaume Lautrette, Fayçal Touti, Hong Geun Lee, Peng Dai, and Bradley L. Pentelute*
Abstract
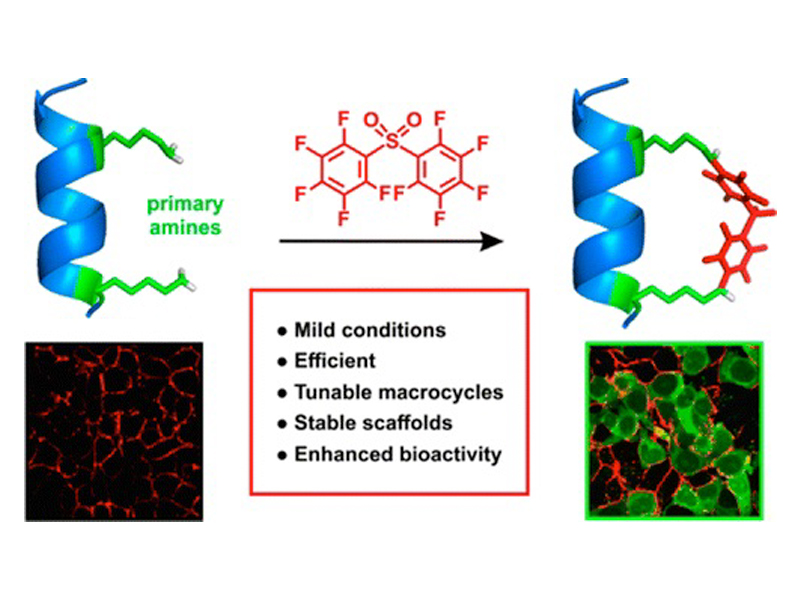
We describe an efficient and mild method for the synthesis of macrocyclic peptides via nitrogen arylation from unprotected precursors. Various electrophiles and lysine-based nucleophiles were investigated and showed high-yielding product formation, even for a macrocyclization scan with 14 variants. We found that nitrogen-linked aryl products were more stable to base and oxidation when compared to thiol arylated species, thereby highlighting the utility of this methodology. Finally, N-aryl macrocyclization was performed on a p53 peptide inhibitor of MDM2 and resulted in identification of a nanomolar binder with improved proteolytic stability and cell permeability.



