
Macrocyclization of Unprotected Peptide Isocyanates
Macrocyclization of Unprotected Peptide Isocyanates
Org. Lett., 2016, 18 (6), pp 1226–1229
DOI: 10.1021/acs.orglett.5b03626
Publication Date (Web): March 7, 2016
Alexander A. Vinogradov, Zi-Ning Choo, Kyle A. Totaro, and Bradley L. Pentelute*
Abstract
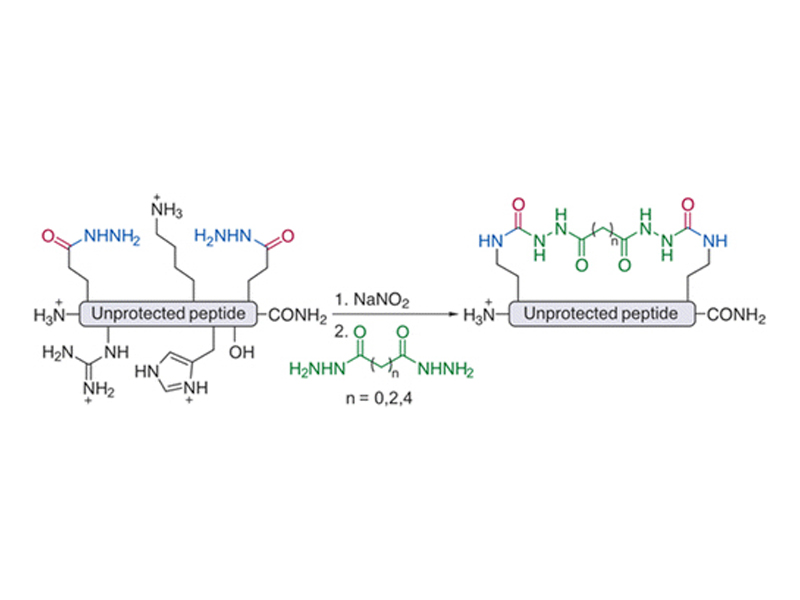
A chemistry for the facile two-component macrocyclization of unprotected peptide isocyanates is described. Starting from peptides containing two glutamic acid γ-hydrazide residues, isocyanates can be readily accessed and cyclized with hydrazides of dicarboxylic acids. The choice of a nucleophilic linker allows for the facile modulation of biochemical properties of a macrocyclic peptide. Four cyclic NYAD-1 analogues were synthesized using the described method and displayed a range of biological activities.



