
Antibody–Bactericidal Macrocyclic Peptide Conjugates To Target Gram‐Negative Bacteria
Antibody–Bactericidal Macrocyclic Peptide Conjugates To Target Gram‐Negative Bacteria
First published: 08 July 2018 https://doi.org/10.1002/cbic.201800295
Dr. Fayçal Touti Dr. Guillaume Lautrette Kenneth D. Johnson Dr. James C. Delaney Dr. Andrew Wollacott Hamid Tissire Dr. Karthik Viswanathan Dr. Zachary Shriver Dr. Surin K. Mong Dr. Alexander J. Mijalis Dr. Obadiah J. Plante Prof. Dr. Bradley L. Pentelute
Abstract
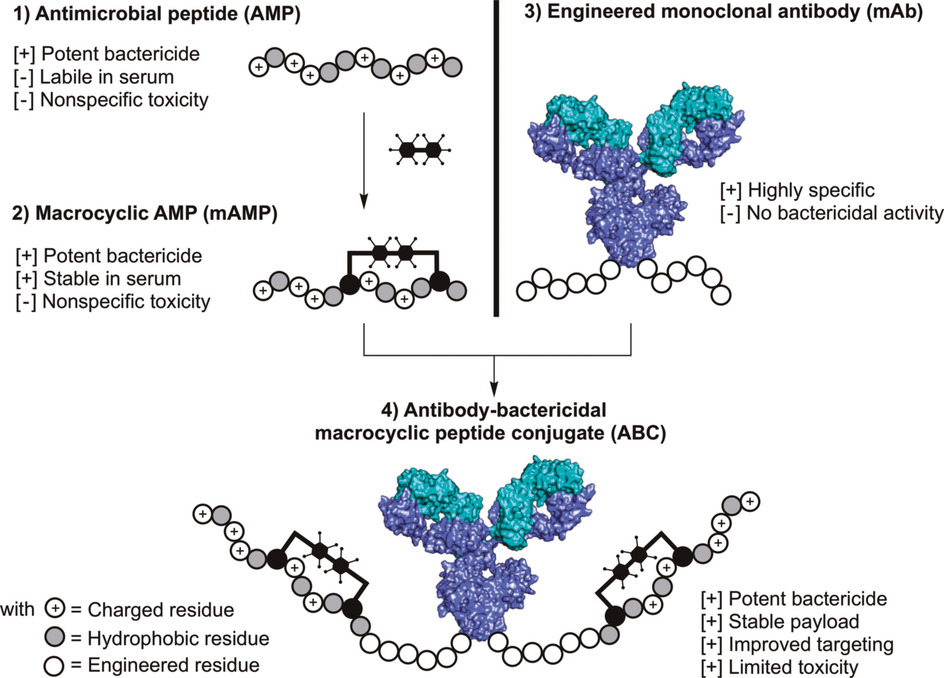
To combat antimicrobial infections, new active molecules are needed. Antimicrobial peptides, ever abundant in nature, are a fertile starting point to develop new antimicrobial agents but suffer from low stability, low specificity, and off‐target toxicity. These drawbacks have limited their development. To overcome some of these limitations, we developed antibody–bactericidal macrocyclic peptide conjugates (ABCs), in which the antibody directs the bioactive macrocyclic peptide to the targeted Gram‐negative bacteria. We used cysteine SNAr chemistry to synthesize and systematically study a library of large (>30‐mer) macrocyclic antimicrobial peptides (mAMPs) to discover variants with extended proteolytic stability in human serum and low hemolytic activity while maintaining bioactivity. We then conjugated, by using sortase A, these bioactive variants onto an Escherichia coli targeted monoclonal antibody. We found that these ABCs had minimized hemolytic activity and were able to kill E. coli at nanomolar concentrations. Our findings suggest macrocyclic peptides if fused to antibodies may facilitate the discovery of new agents to treat bacterial infections.



