
Mutations in pmrB Confer Cross-Resistance between the LptD Inhibitor POL7080 and Colistin in Pseudomonas aeruginosa
Mutations in pmrB Confer Cross-Resistance between the LptD Inhibitor POL7080 and Colistin in Pseudomonas aeruginosa
DOI: 10.1128/AAC.00511-19
Keith P. Romano, Thulasi Warrier, Bradley E. Poulsen, Phuong H. Nguyen, Alexander R. Loftis, Azin Saebi, Bradley L. Pentelute, Deborah T. Hung
Abstract
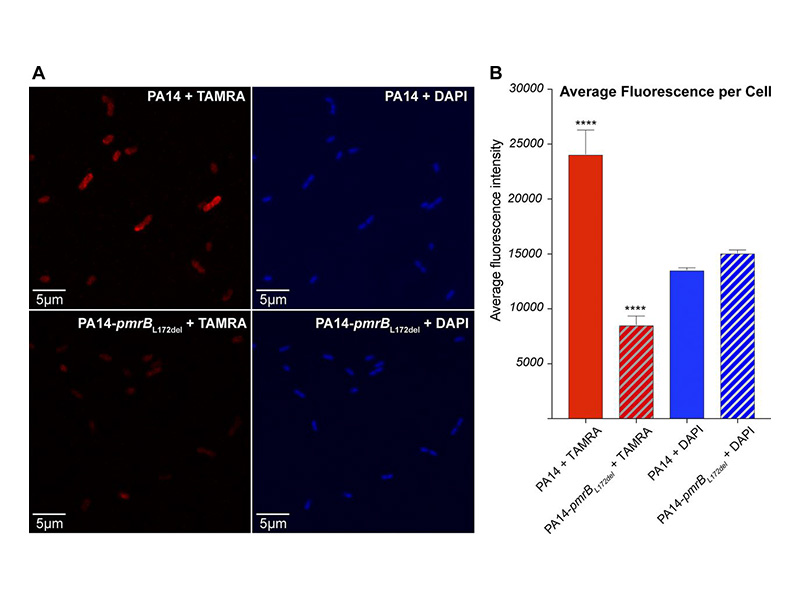
Pseudomonas aeruginosa is a major bacterial pathogen associated with a rising prevalence of antibiotic resistance. We evaluated the resistance mechanisms of P. aeruginosa against POL7080, a species-specific, first-in-class antibiotic in clinical trials that targets the lipopolysaccharide transport protein LptD. We isolated a series of POL7080-resistant strains with mutations in the two-component sensor gene pmrB. Transcriptomic and confocal microscopy studies support a resistance mechanism shared with colistin, involving lipopolysaccharide modifications that mitigate antibiotic cell surface binding.



