
Rapid Total Synthesis of DARPin pE59 and Barnase
Rapid Total Synthesis of DARPin pE59 and Barnase
Mong SK, Vinogradov AA, Simon MD, Pentelute BL., Chembiochem. 2014 Mar 21;15(5):721-33
Published
Online March 11, 2014
Abstract
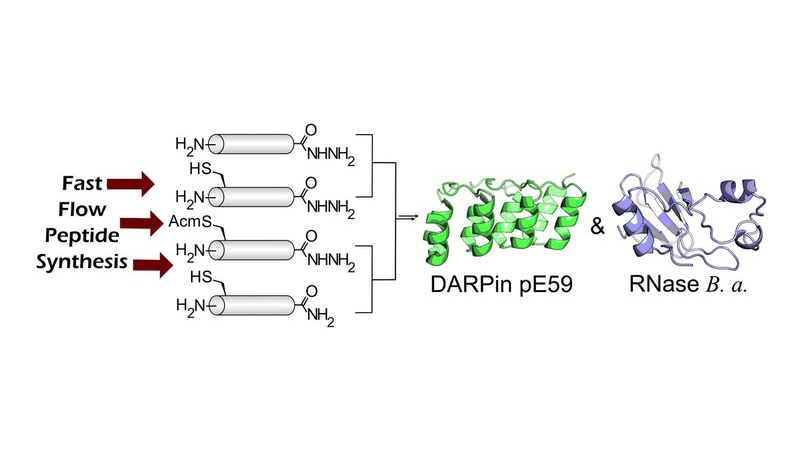
We report the convergent total synthesis of two proteins: DARPin pE59 and Bacillus amyloliquefaciens RNase (Barnase). Leveraging our recently developed fast-flow peptide-synthesis platform, we rapidly explored numerous conditions for the assembly of long polypeptides, and were able to mitigate common side reactions, including deletion and aspartimide products. We report general strategies for improving the synthetic quality of difficult peptide sequences with our system. High-quality protein fragments produced under optimal synthetic conditions were subjected to convergent native chemical ligation, which afforded native full-length proteins after a final desulfurization step. Both DARPin and Barnase were folded and found to be as active as their recombinant analogues.



