Translocation of Non-Canonical Polypeptides into Cells Using Protective Antigen
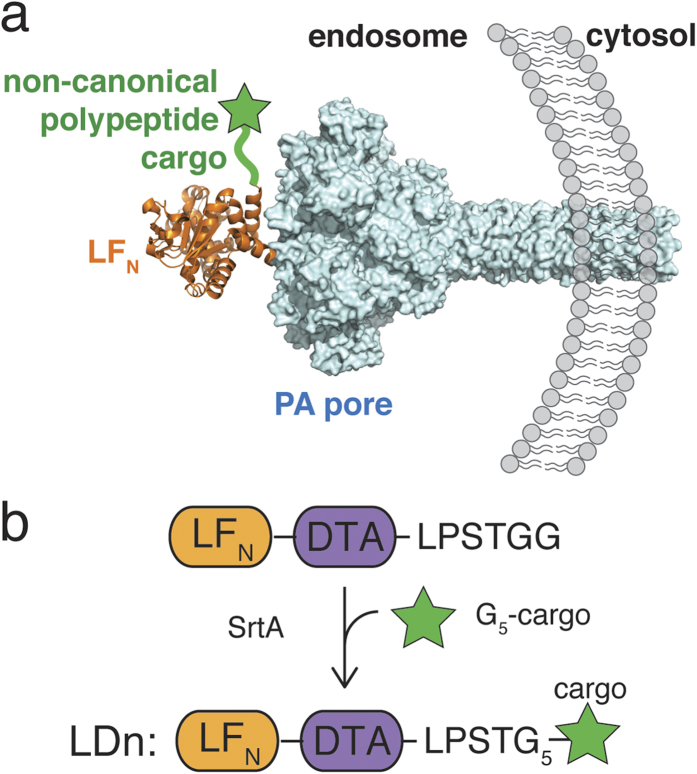
A variety of pathogenic bacteria infect host eukaryotic cells using protein toxins, which enter the cytosol and exert their cytotoxic effects. Anthrax lethal toxin, for example, utilizes the membrane-spanning translocase, protective antigen (PA) pore, to deliver the protein toxin lethal factor (LF) from the endosome into the cytosol of cells. Previous work has investigated the delivery of natural peptides and enzymatic domains appended to the C-terminus of the PA-binding domain of lethal factor (LFN) into the cytosol via PA pore. Here, we move beyond natural amino acids and systematically investigate the translocation of polypeptide cargo containing non-canonical amino acids and functionalities through PA pore. Our results indicate translocation is not perturbed with alterations to the peptide backbone or side-chain. Moreover, despite their structural complexity, we found that the small molecule drugs, doxorubicin and monomethyl auristatin F (MMAF) translocated efficiently through PA pore. However, we found cyclic peptides and the small molecule drug docetaxel abrogated translocation due to their large size and structural rigidity. For cargos that reached the cytosol, we demonstrated that each remained intact after translocation. These studies show PA is capable of translocating non-canonical cargo provided it is in a conformational state conducive for passage through the narrow pore.
Pentelute Lab, MIT, Publications,
15776
wp-singular,portfolio_page-template-default,single,single-portfolio_page,postid-15776,wp-theme-bridge,bridge-core-3.0.1,qode-page-transition-enabled,ajax_fade,page_not_loaded,,paspartu_enabled,paspartu_on_top_fixed,paspartu_on_bottom_fixed,qode_grid_1200,qode_popup_menu_push_text_top,qode-theme-ver-28.7,qode-theme-bridge,disabled_footer_top,wpb-js-composer js-comp-ver-6.8.0,vc_responsive
Translocation of Non-Canonical Polypeptides into Cells Using Protective Antigen
Translocation of Non-Canonical Polypeptides into Cells Using Protective Antigen
Published online 2015 Jul 16
Amy E. Rabideau, Xiaoli Liao, Gizem Akçay, and Bradley L. Pentelutea,
Abstract
A variety of pathogenic bacteria infect host eukaryotic cells using protein toxins, which enter the cytosol and exert their cytotoxic effects. Anthrax lethal toxin, for example, utilizes the membrane-spanning translocase, protective antigen (PA) pore, to deliver the protein toxin lethal factor (LF) from the endosome into the cytosol of cells. Previous work has investigated the delivery of natural peptides and enzymatic domains appended to the C-terminus of the PA-binding domain of lethal factor (LFN) into the cytosol via PA pore. Here, we move beyond natural amino acids and systematically investigate the translocation of polypeptide cargo containing non-canonical amino acids and functionalities through PA pore. Our results indicate translocation is not perturbed with alterations to the peptide backbone or side-chain. Moreover, despite their structural complexity, we found that the small molecule drugs, doxorubicin and monomethyl auristatin F (MMAF) translocated efficiently through PA pore. However, we found cyclic peptides and the small molecule drug docetaxel abrogated translocation due to their large size and structural rigidity. For cargos that reached the cytosol, we demonstrated that each remained intact after translocation. These studies show PA is capable of translocating non-canonical cargo provided it is in a conformational state conducive for passage through the narrow pore.
Category
2015, Publications