
Total synthesis of himastatin
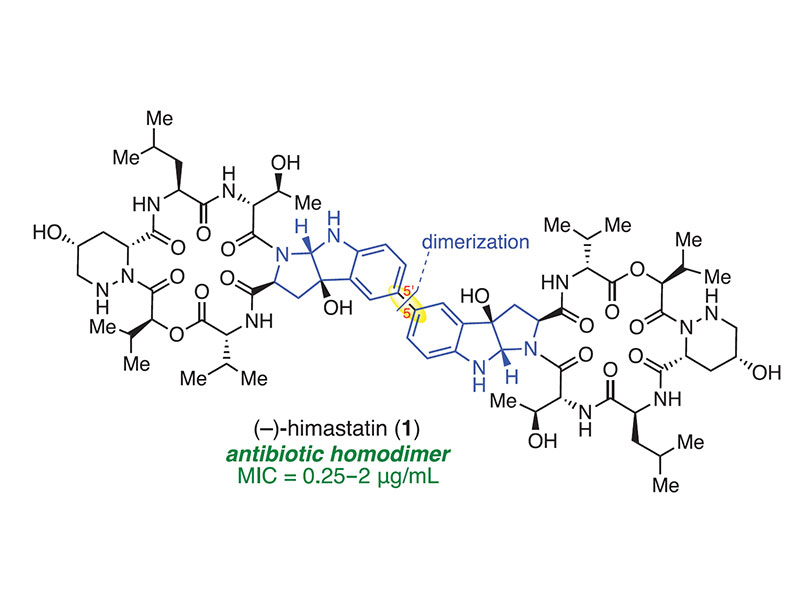
Total synthesis of himastatin
SCIENCE 24 Feb 2022 Vol 375, Issue 6583 pp. 894-899 DOI: 10.1126/science.abm6509
KYAN A. D’ANGELO, CARLY K. SCHISSEL, BRADLEY L. PENTELUTE, AND MOHAMMAD MOVASSAGHI
Abstract
The natural product himastatin has an unusual homodimeric structure that presents a substantial synthetic challenge. We report the concise total synthesis of himastatin from readily accessible precursors, incorporating a final-stage dimerization strategy that was inspired by a detailed consideration of the compound’s biogenesis. Combining this approach with a modular synthesis enabled expedient access to more than a dozen designed derivatives of himastatin, including synthetic probes that provide insight into its antibiotic activity.



