
Total Synthesis and Biochemical Characterization of Mirror Image Barnase
Total Synthesis and Biochemical Characterization of Mirror Image Barnase
Chemical Science (in press, 25 February 2015)
Alexander A. Vinogradova, Ethan D. Evansa, and Bradley L. Pentelute*
Abstract
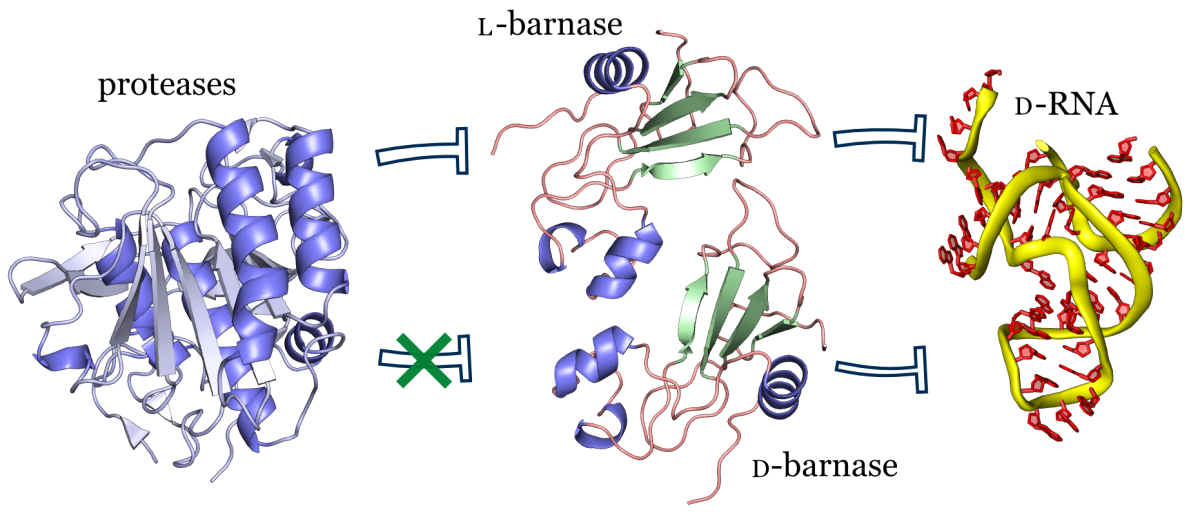
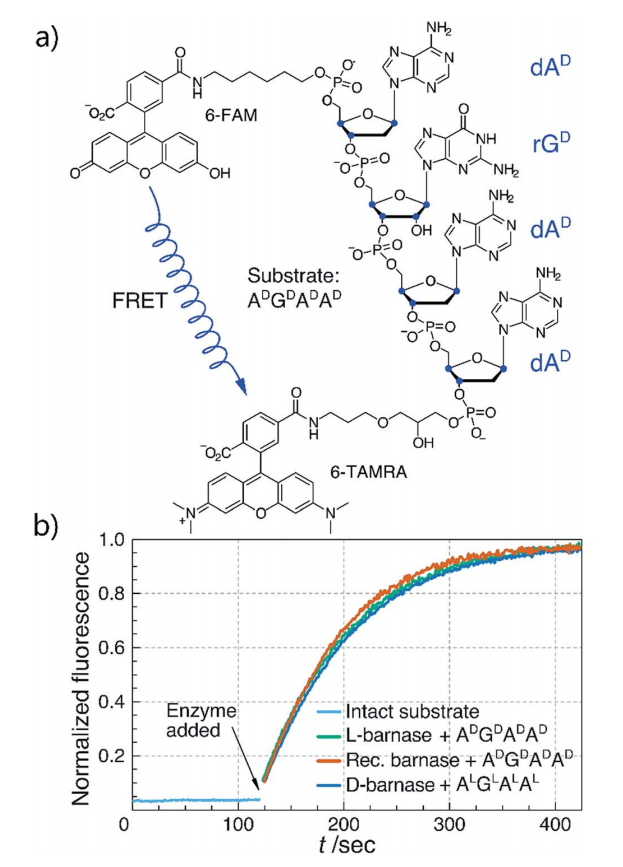
In this study we synthesized and characterized mirror image barnase (B. amyloliquefaciens ribonuclease). D barnase was identical to L-barnase, when analyzed by liquid chromatography and mass-spectrometry. Proteolysis of the mirror image enzyme revealed that in contrast to its native counterpart, D-barnase was completely stable to digestive proteases. In enzymatic assays, D-barnase had the reciprocal chiral specificity and was fully active towards mirror image substrates. Interestingly, D-barnase also hydrolyzed the substrate of the native chirality, albeit 4000 times less efficiently. This effect was further confirmed by digesting a native 112-mer RNA with the enzyme. Additional studies revealed that barnase accommodates a range of substrates with various chiralities, but the prime requirement for guanosine remains. These studies point toward using mirror image enzymes as modern agents in biotechnology.



