Three Dimensional Structure of the Anthrax Toxin Translocon–lethal Factor Complex by Cryo-electron Microscopy
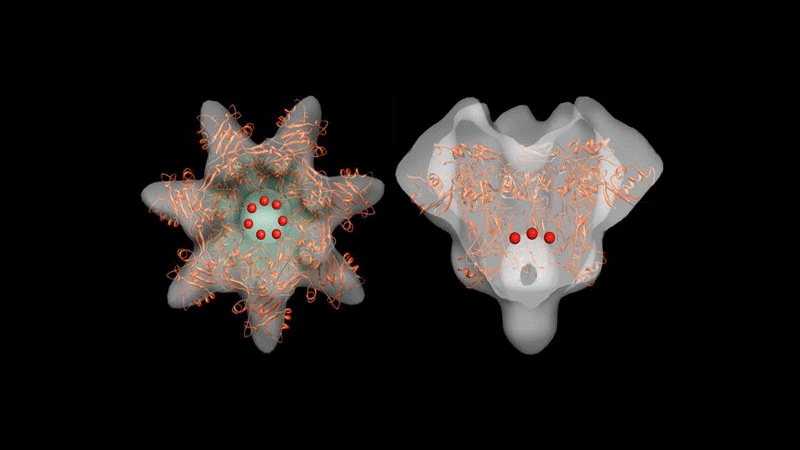
We have visualized by cryo-electron microscopy (cryo-EM) the complex of the anthrax protective antigen (PA) translocon and the N-terminal domain of anthrax lethal factor (LF(N) inserted into a nanodisc model lipid bilayer. We have determined the structure of this complex at a nominal resolution of 16 Å by single-particle analysis and three-dimensional reconstruction. Consistent with our previous analysis of negatively stained unliganded PA, the translocon comprises a globular structure (cap) separated from the nanodisc bilayer by a narrow stalk that terminates in a transmembrane channel (incompletely distinguished in this reconstruction). The globular cap is larger than the unliganded PA pore, probably due to distortions introduced in the previous negatively stained structures. The cap exhibits larger, more distinct radial protrusions, previously identified with PA domain three, fitted by elements of the NMFF PA prepore crystal structure. The presence of LF(N), though not distinguished due to the seven-fold averaging used in the reconstruction, contributes to the distinct protrusions on the cap rim volume distal to the membrane. Furthermore, the lumen of the cap region is less resolved than the unliganded negatively stained PA, due to the low contrast obtained in our images of this specimen. Presence of the LF(N) extended helix and N terminal unstructured regions may also contribute to this additional internal density within the interior of the cap. Initial NMFF fitting of the cryoEM-defined PA pore cap region positions the Phe clamp region of the PA pore translocon directly above an internal vestibule, consistent with its role in toxin translocation.
Pentelute Lab, MIT, Publications, Three Dimensional Structure of the Anthrax Toxin Translocon–lethal Factor Complex by Cryo-electron Microscopy
15738
wp-singular,portfolio_page-template-default,single,single-portfolio_page,postid-15738,wp-theme-bridge,bridge-core-3.0.1,qode-page-transition-enabled,ajax_fade,page_not_loaded,,paspartu_enabled,paspartu_on_top_fixed,paspartu_on_bottom_fixed,qode_grid_1200,qode_popup_menu_push_text_top,qode-theme-ver-28.7,qode-theme-bridge,disabled_footer_top,wpb-js-composer js-comp-ver-6.8.0,vc_responsive
Three Dimensional Structure of the Anthrax Toxin Translocon–lethal Factor Complex by Cryo-electron Microscopy
Three Dimensional Structure of the Anthrax Toxin Translocon–lethal Factor Complex by Cryo-electron Microscopy
Gogol, Akkaladevi, Szerszen, Mukherjee, Chollet-Hinton, Katayama, Pentelute, Collier, Fisher, Protein Sci., 2013, 22, 586-94.
Published
Online March 13 2013
Abstract
We have visualized by cryo-electron microscopy (cryo-EM) the complex of the anthrax protective antigen (PA) translocon and the N-terminal domain of anthrax lethal factor (LF(N) inserted into a nanodisc model lipid bilayer. We have determined the structure of this complex at a nominal resolution of 16 Å by single-particle analysis and three-dimensional reconstruction. Consistent with our previous analysis of negatively stained unliganded PA, the translocon comprises a globular structure (cap) separated from the nanodisc bilayer by a narrow stalk that terminates in a transmembrane channel (incompletely distinguished in this reconstruction). The globular cap is larger than the unliganded PA pore, probably due to distortions introduced in the previous negatively stained structures. The cap exhibits larger, more distinct radial protrusions, previously identified with PA domain three, fitted by elements of the NMFF PA prepore crystal structure. The presence of LF(N), though not distinguished due to the seven-fold averaging used in the reconstruction, contributes to the distinct protrusions on the cap rim volume distal to the membrane. Furthermore, the lumen of the cap region is less resolved than the unliganded negatively stained PA, due to the low contrast obtained in our images of this specimen. Presence of the LF(N) extended helix and N terminal unstructured regions may also contribute to this additional internal density within the interior of the cap. Initial NMFF fitting of the cryoEM-defined PA pore cap region positions the Phe clamp region of the PA pore translocon directly above an internal vestibule, consistent with its role in toxin translocation.
Category
2013, Publications