
Systematic Investigation of EDC/sNHS-Mediated Bioconjugation Reactions for Carboxylated Peptide Substrates
Systematic Investigation of EDC/sNHS-Mediated Bioconjugation Reactions for Carboxylated Peptide Substrates
Bioconjugate Chem., 2016, 27 (4), pp 994–1004
DOI: 10.1021/acs.bioconjchem.6b00043
Publication Date (Web): March 14, 2016
Kyle A. Totaro†, Xiaoli Liao†, Keshab Bhattacharya‡, Jari I. Finneman‡, Justin B. Sperry‡, Mark A. Massa‡, Jennifer Thorn‡, Sa V. Ho§, and Bradley L. Pentelute*†
Abstract
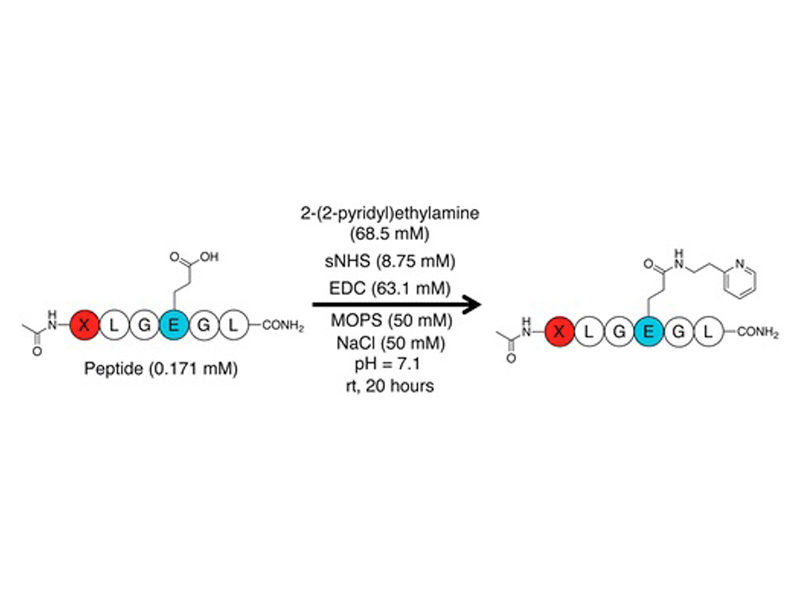
1-Ethyl-3-(3-(dimethylamino)propyl)carbodiimide (EDC) bioconjugations have been utilized in preparing variants for medical research. While there have been advances in optimizing the reaction for aqueous applications, there has been limited focus toward identifying conditions and side reactions that interfere with product formation. We present a systematic investigation of EDC/N-hydroxysulfosuccinimide (sNHS)-mediated bioconjugations on carboxylated peptides and small proteins. We identified yet-to-be-reported side products arising from both the reagents and substrates. Model peptides used in this study illustrate particular substrates are more susceptible to side reactions than others. From our studies, we found that bioconjugations are more efficient with high concentrations of amine nucleophile but not sNHS. Performing bioconjugations on a model affibody protein show that the trends established with model peptides hold for more complex systems.



