
Synthesis of proteins by automated flow chemistry
Synthesis of proteins by automated flow chemistry
Science 29 May 2020:
Vol. 368, Issue 6494, pp. 980-987
DOI: 10.1126/science.abb2491
N. Hartrampf, A. Saebi1, ProfileM. Poskus1, Z. P. Gates1, A. J. Callahan, A. E. Cowfer, S. Hanna, S. Antilla, ProfileC. K. Schissel, ProfileA. J. Quartararo1, X. Ye, A. J. Mijalis, M. D. Simon1, A. Loas, S. Liu, ProfileC. Jessen, T. E. Nielsen, B. L. Pentelute
Abstract
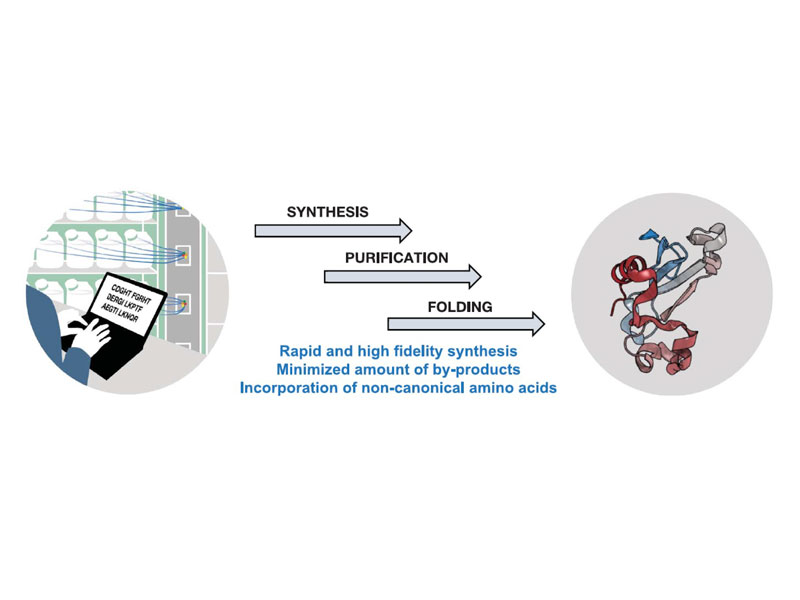
Ribosomes can produce proteins in minutes and are largely constrained to proteinogenic amino acids. Here, we report highly efficient chemistry matched with an automated fast-flow instrument for the direct manufacturing of peptide chains up to 164 amino acids long over 327 consecutive reactions. The machine is rapid: Peptide chain elongation is complete in hours. We demonstrate the utility of this approach by the chemical synthesis of nine different protein chains that represent enzymes, structural units, and regulatory factors. After purification and folding, the synthetic materials display biophysical and enzymatic properties comparable to the biologically expressed proteins. High-fidelity automated flow chemistry is an alternative for producing single-domain proteins without the ribosome.



