
Secondary Amino Alcohols: Traceless Cleavable Linkers for Use in Affinity Capture and Release
Secondary Amino Alcohols: Traceless Cleavable Linkers for Use in Affinity Capture and Release
First published: 29 March 2020 https://doi.org/10.1002/anie.202003478
Dr. Sebastian Pomplun, Dr. Christopher R. Shugrue, Adeline M. Schmitt, Carly K. Schissel, Charlotte E. Farquhar, Prof. Dr. Bradley L. Pentelute
Abstract
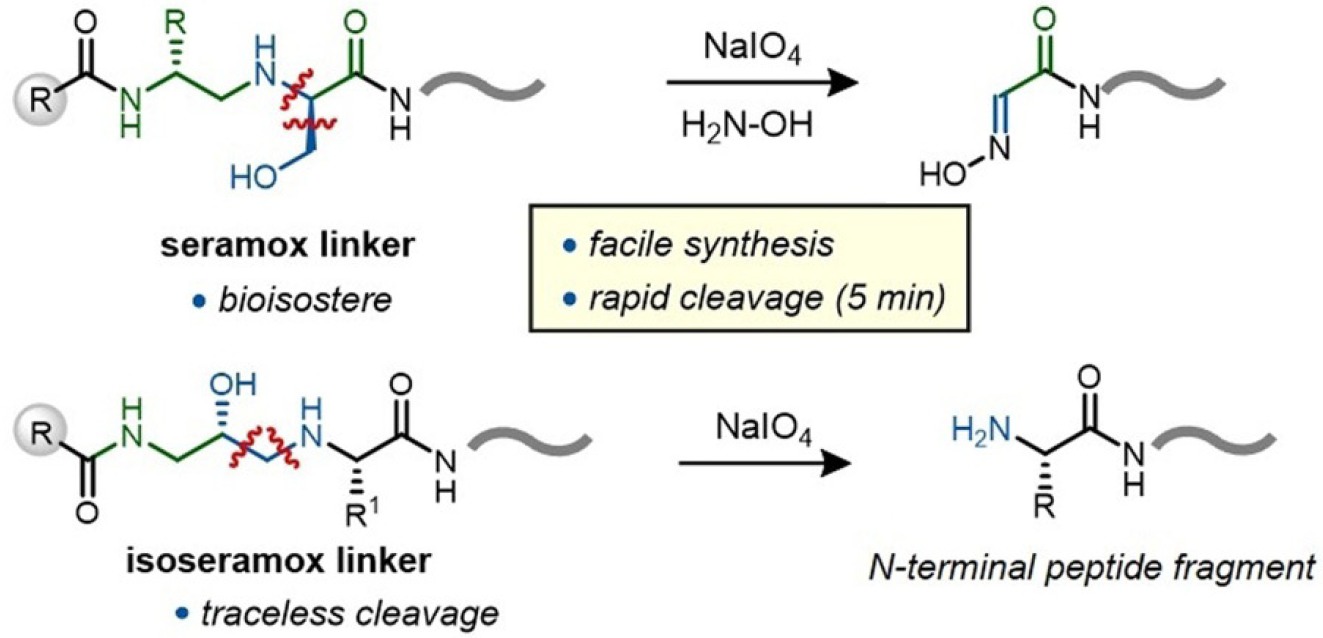
Capture and release of peptides is often a critical operation in the pathway to discovering materials with novel functions. However, the best methods for efficient capture impede facile release. To overcome this challenge, we report linkers based on secondary amino alcohols for the release of peptides after capture. These amino alcohols are based on serine (seramox) or isoserine (isoseramox) and can be incorporated into peptides during solid-phase peptide synthesis through reductive amination. Both linkers are quantitatively cleaved within minutes under NaIO4 treatment. Cleavage of isoseramox produced a native peptide N-terminus. This linker also showed broad substrate compatibility; incorporation into a synthetic peptide library resulted in the identification of all sequences by nanoLC-MS/MS. The linkers are cell compatible; a cell-penetrating peptide that contained this linker was efficiently captured and identified after uptake into cells. These findings suggest that such secondary amino alcohol based linkers might be suitable tools for peptide-discovery platforms.



