
Palladium–Protein Oxidative Addition Complexes by Amine-Selective Acylation
Palladium–Protein Oxidative Addition Complexes by Amine-Selective Acylation
J. Am. Chem. Soc. 2020, 142, 51, 21237–21242
Publication Date:December 15, 2020
https://doi.org/10.1021/jacs.0c09180
Heemal H. Dhanjee, Ivan Buslov, Ian W. Windsor, Ronald T. Raines, Bradley L. Pentelute*, and Stephen L. Buchwald
Abstract
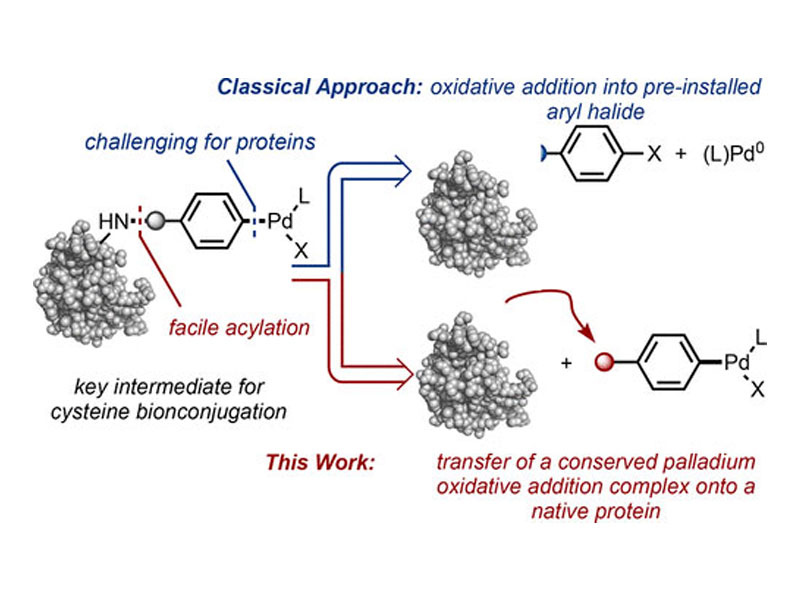
Palladium oxidative addition complexes (OACs) are traditionally accessed by treating an aryl halide-containing substrate with a palladium(0) source. Here, a new strategy to selectively prepare stable OACs from amino groups on native proteins is presented. The approach relies on an amine-selective acylation reaction that occurs without modification of a preformed palladium(II)-aryl group. Once transferred onto a protein substrate, the palladium(II)-aryl group facilitates conjugation by undergoing reaction with a second, cysteine-containing protein. This operationally simple method is applicable to native, nonengineered enzymes as well as antibodies and is carried out in an aqueous setting and open to air. The resulting Pd–protein OACs are stable, storable reagents that retain biological activity and can be used to achieve protein–protein cross-coupling at nanomolar concentrations within hours.



