
Palladium Mediated Synthesis of Protein–Polyarene Conjugates
Palladium Mediated Synthesis of Protein–Polyarene Conjugates
J. Am. Chem. Soc. 2022, 144, 26, 11706–11712, Publication Date:June 24, 2022
Jacob Rodriguez, Heemal H. Dhanjee, Bradley L. Pentelute*, and Stephen L. Buchwald.
Abstract
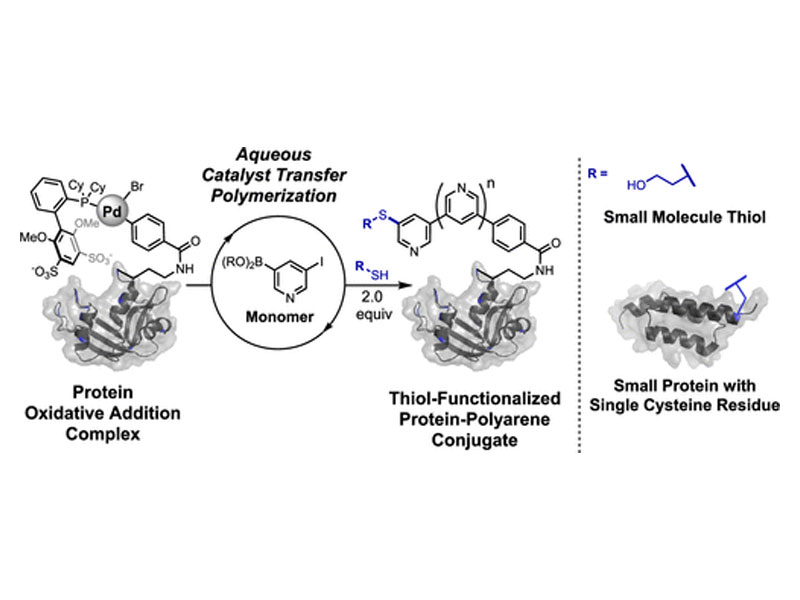
Catalyst transfer polymerization (CTP) is widely applied to the synthesis of well-defined π-conjugated polymers. Unlike other polymerization reactions that can be performed in water (e.g., controlled radical polymerizations and ring-opening polymerizations), CTP has yet to be adapted for the modification of biopolymers. Here, we report the use of protein–palladium oxidative addition complexes (OACs) that enable catalyst transfer polymerization to furnish protein–polyarene conjugates. These polymerizations occur with electron-deficient monomers in aqueous buffers open to air at mild (≤37 °C) temperatures with full conversion of the protein OAC and an average polymer length of nine repeating units. Proteins with polyarene chains terminated with palladium OACs can be readily isolated. Direct evidence of protein–polyarene OAC formation was obtained using mass spectrometry, and all protein–polyarene chain ends were uniformly functionalized via C–S arylation to terminate the polymerization with a small molecule thiol or a cysteine-containing protein.



