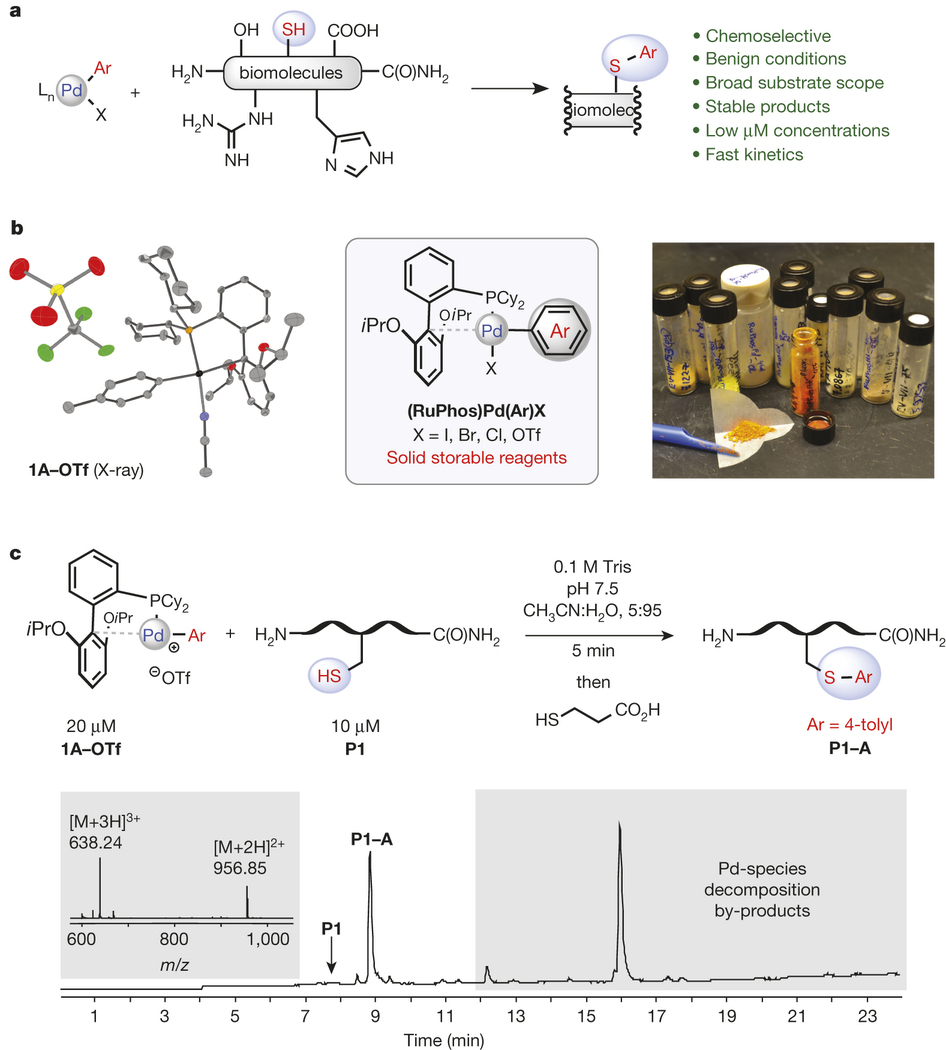
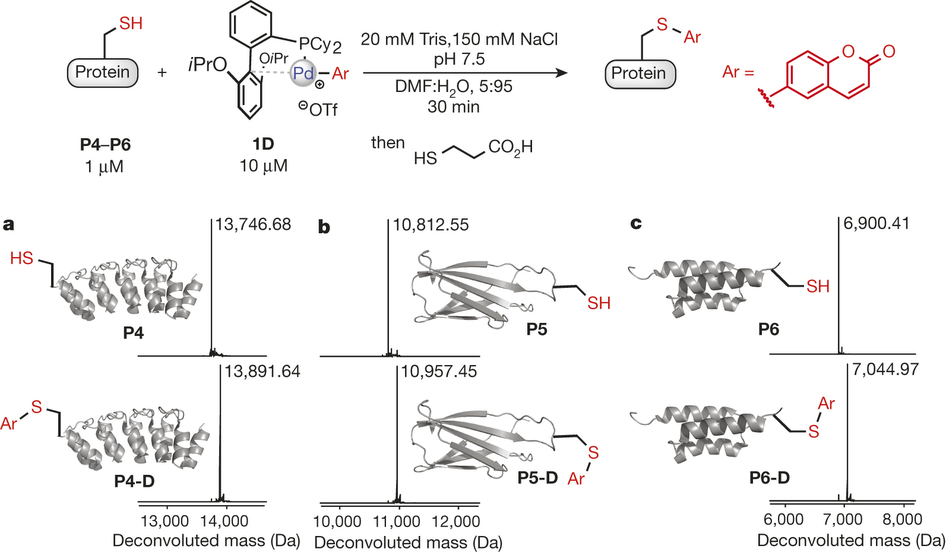
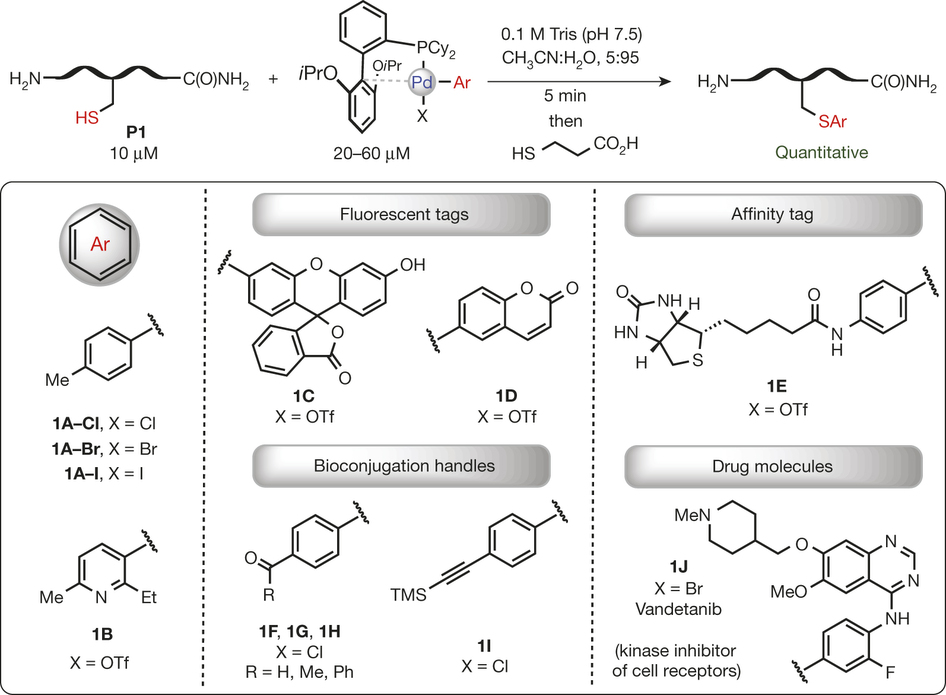
Organometallic palladium reagents for cysteine bioconjugation
Reactions based on transition metals have found wide use in organic synthesis, in particular for the functionalization of small molecules1, 2. However, there are very few reports of using transition-metal-based reactions to modify complex biomolecules3, 4, which is due to the need for stringent reaction conditions (for example, aqueous media, low temperature and mild pH) and the existence of multiple reactive functional groups found in biomolecules. Here we report that palladium(II) complexes can be used for efficient and highly selective cysteine conjugation (bioconjugation) reactions that are rapid and robust under a range of bio-compatible reaction conditions. The straightforward synthesis of the palladium reagents from diverse and easily accessible aryl halide and trifluoromethanesulfonate precursors makes the method highly practical, providing access to a large structural space for protein modification. The resulting aryl bioconjugates are stable towards acids, bases, oxidants and external thiol nucleophiles. The broad utility of the bioconjugation platform was further corroborated by the synthesis of new classes of stapled peptides and antibody–drug conjugates. These palladium complexes show potential as benchtop reagents for diverse bioconjugation applications.
Pentelute Lab, MIT, Publications,
15780
wp-singular,portfolio_page-template-default,single,single-portfolio_page,postid-15780,wp-theme-bridge,bridge-core-3.0.1,qode-page-transition-enabled,ajax_fade,page_not_loaded,,paspartu_enabled,paspartu_on_top_fixed,paspartu_on_bottom_fixed,qode_grid_1200,qode_popup_menu_push_text_top,qode-theme-ver-28.7,qode-theme-bridge,disabled_footer_top,wpb-js-composer js-comp-ver-6.8.0,vc_responsive
Organometallic palladium reagents for cysteine bioconjugation
Organometallic palladium reagents for cysteine bioconjugation
Published on 28 October 2015
Ekaterina V. Vinogradova, Chi Zhang, Alexander M. Spokoyny, Bradley L. Pentelute & Stephen L. Buchwald
Abstract
Reactions based on transition metals have found wide use in organic synthesis, in particular for the functionalization of small molecules1, 2. However, there are very few reports of using transition-metal-based reactions to modify complex biomolecules3, 4, which is due to the need for stringent reaction conditions (for example, aqueous media, low temperature and mild pH) and the existence of multiple reactive functional groups found in biomolecules. Here we report that palladium(II) complexes can be used for efficient and highly selective cysteine conjugation (bioconjugation) reactions that are rapid and robust under a range of bio-compatible reaction conditions. The straightforward synthesis of the palladium reagents from diverse and easily accessible aryl halide and trifluoromethanesulfonate precursors makes the method highly practical, providing access to a large structural space for protein modification. The resulting aryl bioconjugates are stable towards acids, bases, oxidants and external thiol nucleophiles. The broad utility of the bioconjugation platform was further corroborated by the synthesis of new classes of stapled peptides and antibody–drug conjugates. These palladium complexes show potential as benchtop reagents for diverse bioconjugation applications.
Category
2015, Publications