
In-Cell Penetration Selection–Mass Spectrometry Produces Noncanonical Peptides for Antisense Delivery
In-Cell Penetration Selection–Mass Spectrometry Produces Noncanonical Peptides for Antisense Delivery
Carly K. Schissel, Charlotte E. Farquhar, Andrei Loas, Annika B. Malmberg, and Bradley L. Pentelute
Abstract
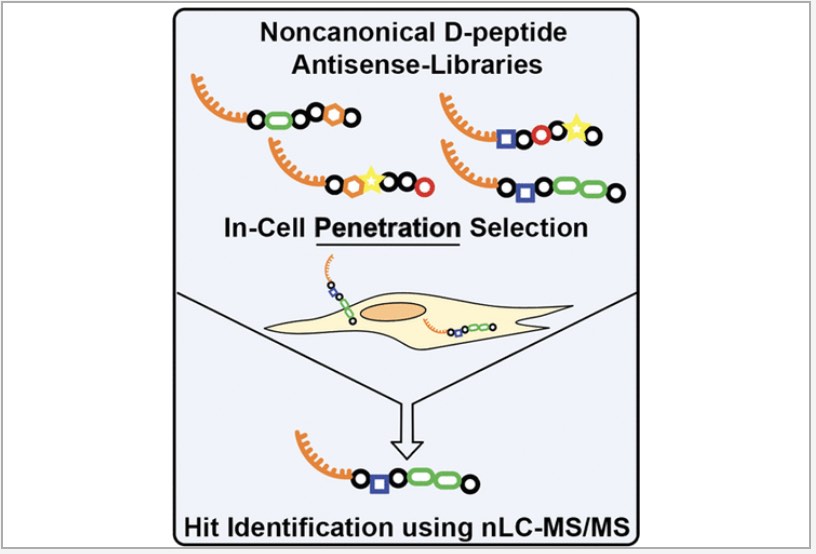
Peptide-mediated delivery of macromolecules in cells has significant potential therapeutic benefits, but no therapy employing cell-penetrating peptides (CPPs) has reached the market after 30 years of investigation due to challenges in the discovery of new, more efficient sequences. Here, we demonstrate a method for in-cell penetration selection–mass spectrometry (in-cell PS–MS) to discover peptides from a synthetic library capable of delivering macromolecule cargo to the cytosol. This method was inspired by recent in vivo selection approaches for cell-surface screening, with an added spatial dimension resulting from subcellular fractionation. A representative peptide discovered in the cytosolic extract, Cyto1a, is nearly 100-fold more active toward antisense phosphorodiamidate morpholino oligomer (PMO) delivery compared to a sequence identified from a whole cell extract, which includes endosomes. Cyto1a is composed of d-residues and two non-α-amino acids, is more stable than its all-l isoform, and is less toxic than known CPPs with comparable activity. Pulse-chase and microscopy experiments revealed that while the PMO–Cyto1a conjugate is likely taken up by endosomes, it can escape to localize to the nucleus without nonspecifically releasing other endosomal components. In-cell PS–MS introduces a means to empirically discover unnatural synthetic peptides for subcellular delivery of therapeutically relevant cargo.



