
Enzyme-Catalyzed Macrocyclization of Long Unprotected Peptides
Enzyme-Catalyzed Macrocyclization of Long Unprotected Peptides
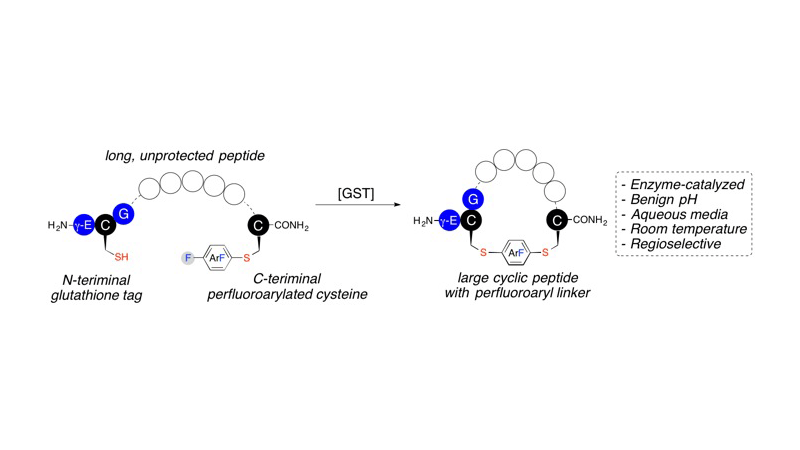
Zhang C, Dai P, Spokoyny AM, Pentelute BL., Org Lett. 2014 Jul 18;16(14):3652-5
Published
Online July 8, 2014
Abstract
A glutathione S-transferase (GST) catalyzed macrocyclization reaction for peptides up to 40 amino acids in length is reported. GST catalyzes the selective S(N)Ar reaction between an N-terminal glutathione (GSH, γ-Glu-Cys-Gly) tag and a C-terminal perfluoroaryl-modified cysteine on the same polypeptide chain. Cyclic peptides ranging from 9 to 24 residues were quantitatively produced within 2 h in aqueous pH = 8 buffer at room temperature. The reaction was highly selective for cyclization at the GSH tag, enabling the combination of GST-catalyzed ligation with native chemical ligation to generate a large 40-residue peptide macrocycle.



