
De Novo Discovery of High-Affinity Peptide Binders for the SARS-CoV-2 Spike Protein
De Novo Discovery of High-Affinity Peptide Binders for the SARS-CoV-2 Spike Protein
ACS Cent. Sci. 2021, 7, 1, 156–163
Publication Date:December 17, 2020
https://doi.org/10.1021/acscentsci.0c01309
Sebastian Pomplun, Muhammad Jbara, Anthony J. Quartararo, Genwei Zhang, Joseph S. Brown, Yen-Chun Lee, Xiyun Ye, Stephanie Hanna, and Bradley L. Pentelute
Abstract
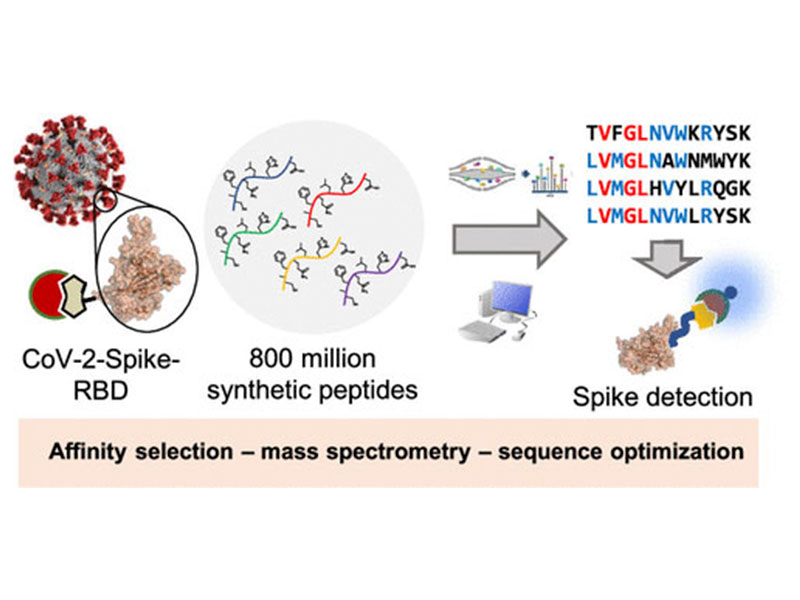
The β-coronavirus SARS-CoV-2 has caused a global pandemic. Affinity reagents targeting the SARS-CoV-2 spike protein are of interest for the development of therapeutics and diagnostics. We used affinity selection–mass spectrometry for the rapid discovery of synthetic high-affinity peptide binders for the receptor binding domain (RBD) of the SARS-CoV-2 spike protein. From library screening with 800 million synthetic peptides, we identified three sequences with nanomolar affinities (dissociation constants Kd = 80–970 nM) for RBD and selectivity over human serum proteins. Nanomolar RBD concentrations in a biological matrix could be detected using the biotinylated lead peptide in ELISA format. These peptides do not compete for ACE2 binding, and their site of interaction on the SARS-CoV-2-spike-RBD might be unrelated to the ACE2 binding site, making them potential orthogonal reagents for sandwich immunoassays. These findings serve as a starting point for the development of SARS-CoV-2 diagnostics or conjugates for virus-directed delivery of therapeutics.



