
Conformational Dynamics in Extended RGD-Containing Peptides
Conformational Dynamics in Extended RGD-Containing Peptides
Biomacromolecules 2020, 21, 7, 2786–2794
Publication Date:May 29, 2020
https://doi.org/10.1021/acs.biomac.0c00506
William R. Lindemann, Alexander J. Mijalis, José L. Alonso, Peter P. Borbat, Jack H. Freed, M. Amin Arnaout, Bradley L. Pentelute, and Julia H. Ortony
Abstract
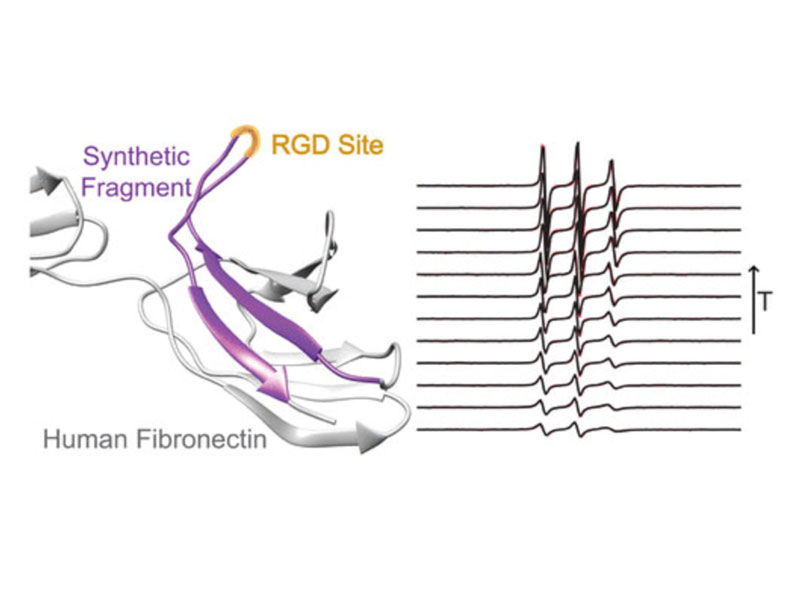
RGD is a prolific example of a tripeptide used in biomaterials for cell adhesion, but the potency of free or surface-bound RGD tripeptide is orders-of-magnitude less than the RGD domain within natural proteins. We designed a set of peptides with varying lengths, composed of fragments of fibronectin protein whose central three residues are RGD, in order to vary their conformational behavior without changing the binding site’s chemical environment. With these peptides, we measure the conformational dynamics and transient structure of the active site. Our studies reveal how flanking residues affect conformational behavior and integrin binding. We find that disorder of the binding site is important to the potency of RGD peptides and that transient hydrogen bonding near the RGD site affects both the energy landscape roughness of the peptides and peptide binding. This phenomenon is independent of longer-range folding interactions and helps explain why short binding sequences, including RGD itself, do not fully replicate the integrin-targeting properties of extracellular matrix proteins. Our studies reinforce that peptide binding is a holistic event and fragments larger than those directly involved in binding should be considered in the design of peptide epitopes for functional biomaterials.



