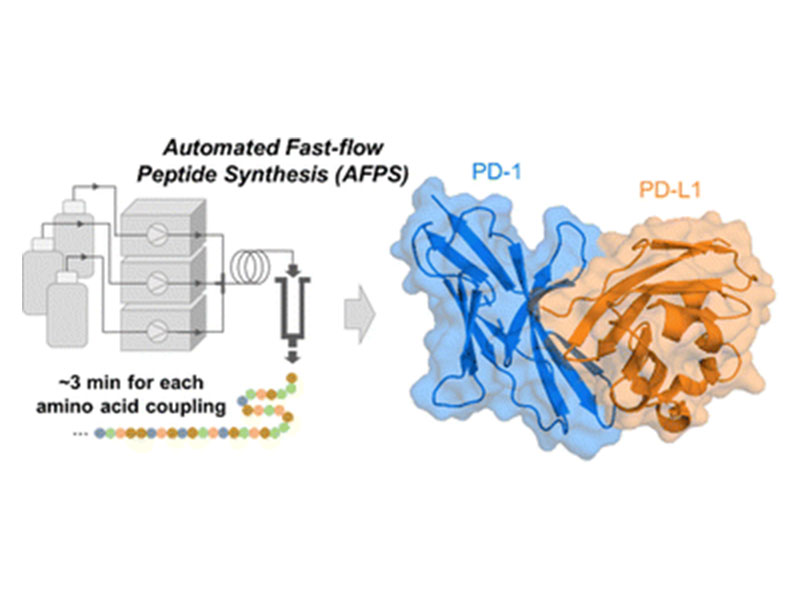
Automated fast-flow synthesis of the immune checkpoint receptors PD-1 and PD-L1
The Pentelute Lab aims to invent new chemistry for the efficient and selective modification of proteins, to ‘hijack’ these biological machines for efficient drug delivery into cells and to create new machines to rapidly and efficiently manufacture peptides and proteins.
Pentelute Lab, Chemistry, MIT, Chemistry Department, Boston, Cambridge, Biology, Peptides, Peptide, Proteins, Science, Rapid, Brad Pentelute, Brad,
18351
wp-singular,portfolio_page-template-default,single,single-portfolio_page,postid-18351,wp-theme-bridge,bridge-core-3.0.1,qode-page-transition-enabled,ajax_fade,page_not_loaded,,paspartu_enabled,paspartu_on_top_fixed,paspartu_on_bottom_fixed,qode_grid_1200,qode_popup_menu_push_text_top,qode-theme-ver-28.7,qode-theme-bridge,disabled_footer_top,wpb-js-composer js-comp-ver-6.8.0,vc_responsive
Automated fast-flow synthesis of the immune checkpoint receptors PD-1 and PD-L1
Automated fast-flow synthesis of the immune checkpoint receptors PD-1 and PD-L1
Giulio Fittolani, Alex J. Callahan, Andrei Loas and Bradley L. Pentelute
Abstract
Programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) are key targets for cancer therapy. Here, we use automated fast-flow peptide synthesis (AFPS) to rapidly produce these challenging β-sheet-rich proteins in their active forms following oxidative refolding protocols. The methods presented here provide rapid access to synthetic, air-stable mutants of PD-1 and PD-L1 in which L-methionine residues are substituted with L-norleucine, potentially enabling investigation of post-translational modifications and mirror-image analogs for drug discovery.
Category
2025, Publications