
A novel, safe, fast and efficient treatment for Her2‐positive and negative bladder cancer utilizing an EGF‐anthrax toxin chimera
A novel, safe, fast and efficient treatment for Her2‐positive and negative bladder cancer utilizing an EGF‐anthrax toxin chimera
IJC 04 October 2019 https://doi.org/10.1002/ijc.32719
Sherwin Jack Kayalvizhi Madhivanan Swetha Ramadesikan Sneha Subramanian Daniel F. Edwards II Bennett D. Elzey Deepika Dhawan Andrew McCluskey Erin M. Kischuk Alexander R. Loftis Nicholas Truex Michael Santos Mike Lu Amy Rabideau Bradley Pentelute John Collier Hristos Kaimakliotis Michael Koch Timothy L. Ratliff Deborah W. Knapp Ruben C. Aguilar
Abstract
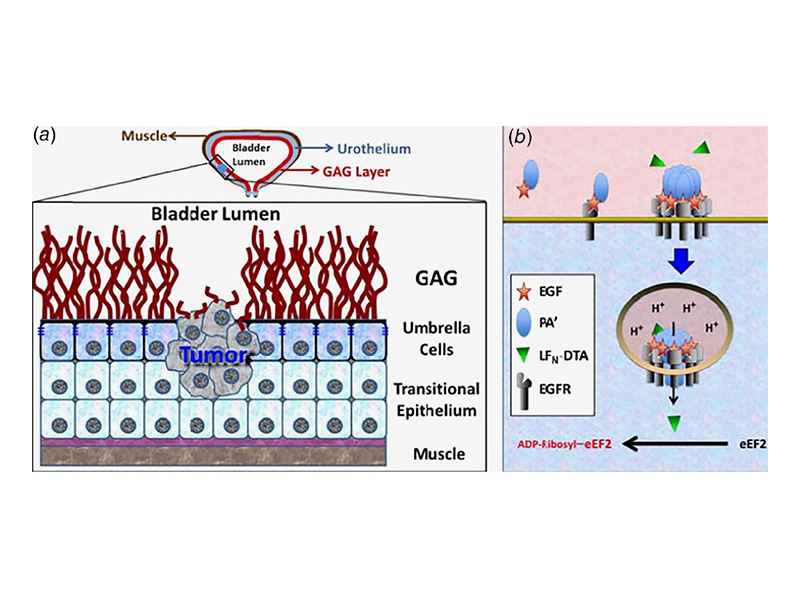
Bladder cancer is the sixth most common cancer in the United States, and it exhibits an alarming 70% recurrence rate. Thus, the development of more efficient antibladder cancer approaches is a high priority. Accordingly, this work provides the basis for a transformative anticancer strategy that takes advantage of the unique characteristics of the bladder. Unlike mucin‐shielded normal bladder cells, cancer cells are exposed to the bladder lumen and overexpress EGFR. Therefore, we used an EGF‐conjugated anthrax toxin that after targeting EGFR was internalized and triggered apoptosis in exposed bladder cancer cells. This unique agent presented advantages over other EGF‐based technologies and other toxin‐derivatives. In contrast to known agents, this EGF‐toxin conjugate promoted its own uptake via receptor microclustering even in the presence of Her2 and induced cell death with a LC50 < 1 nM. Furthermore, our data showed that exposures as short as ≈3 min were enough to commit human (T24), mouse (MB49) and canine (primary) bladder cancer cells to apoptosis. Exposure of tumor‐free mice and dogs with the agent resulted in no toxicity. In addition, the EGF‐toxin was able to eliminate cells from human patient tumor samples. Importantly, the administration of EGF‐toxin to dogs with spontaneous bladder cancer, who had failed or were not eligible for other therapies, resulted in ~30% average tumor reduction after one treatment cycle. Because of its in vitro and in vivo high efficiency, fast action (reducing treatment time from hours to minutes) and safety, we propose that this EGF–anthrax toxin conjugate provides the basis for new, transformative approaches against bladder cancer.



