
Chemical Synthesis, Refolding, and Characterization of Mirror-Image Cyclophilin A
Chemical Synthesis, Refolding, and Characterization of Mirror-Image Cyclophilin A
Ahmet Yesilcimen Satish Gandhesiri Tara L. Travaline Alex J. Callahan Olena S. Tokareva Andrei Loas John H. McGee* Bradley L. Pentelute
Abstract
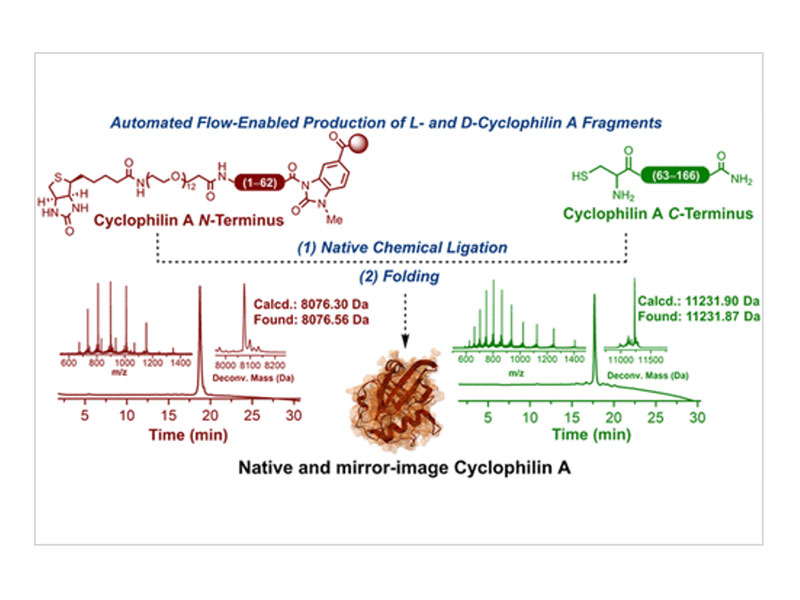
The chemical synthesis of proteins (CSP) has been an essential tool in studying and understanding the role of these biological polymers and in enabling the discovery of novel classes of inhibitors. However, CSP with commercially available synthesizers is typically limited to producing polypeptides of about 50 to 70 amino acids in length. Consequently, a wide range of protein targets have been inaccessible using these technologies, or they require cumbersome synthesis and purification of multiple peptide fragments. In this report, we employed a powerful combination of automated fast-flow peptide synthesis (AFPS), native chemical ligation (NCL), and high-throughput evaluation of refolding conditions to achieve the first chemical synthesis of both the wild-type and mirror-image forms of functional full-length cyclophilin A, which plays a vital role in proline cis–trans isomerization and other important processes. Functional assays confirmed that the chemically synthesized proteins retained their biological properties.



