
Reversibly Reactive Affinity Selection–Mass Spectrometry Enables Identification of Covalent Peptide Binders
Reversibly Reactive Affinity Selection–Mass Spectrometry Enables Identification of Covalent Peptide Binders
Peiyuan ZhangXiyun YeJohn C. K. WangHannah T. BaddockZena JensvoldIan T. FoeAndrei LoasDan L. EatonQi HaoAaron H. Nile*Bradley L. Pentelute*
Abstract
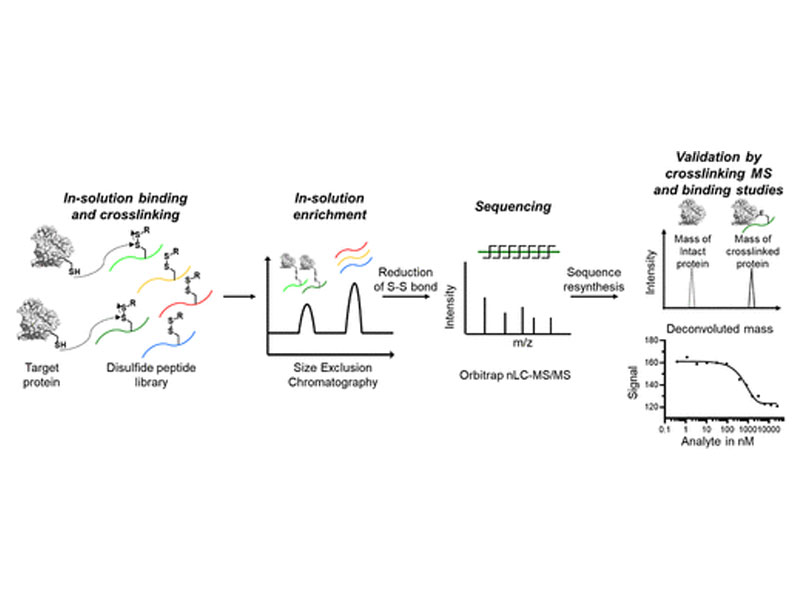
Covalent peptide binders have found applications as activity-based probes and as irreversible therapeutic inhibitors. Currently, there is no rapid, label-free, and tunable affinity selection platform to enrich covalent reactive peptide binders from synthetic libraries. We address this challenge by developing a reversibly reactive affinity selection platform termed ReAct-ASMS enabled by tandem high-resolution mass spectrometry (MS/MS) to identify covalent peptide binders to native protein targets. It uses mixed disulfide-containing peptides to build reversible peptide–protein conjugates that can enrich for covalent variants, which can be sequenced by MS/MS after reduction. Using this platform, we identified covalent peptide binders against two oncoproteins, human papillomavirus 16 early protein 6 (HPV16 E6) and peptidyl-prolyl cis–trans isomerase NIMA-interacting 1 protein (Pin1). The resulting peptide binders efficiently and selectively cross-link Cys58 of E6 at 37 °C and Cys113 of Pin1 at room temperature, respectively. ReAct-ASMS enables the identification of highly selective covalent peptide binders for diverse molecular targets, introducing an applicable platform to assist preclinical therapeutic development pipelines.



