
Parallel Automated Flow Synthesis of Covalent Protein Complexes That Can Inhibit MYC-Driven Transcription
Parallel Automated Flow Synthesis of Covalent Protein Complexes That Can Inhibit MYC-Driven Transcription
CS Cent. Sci. 2021, 7, 8, 1408–1418
Publication Date:August 4, 2021
https://doi.org/10.1021/acscentsci.1c00663
Sebastian Pomplun, Muhammad Jbara, Carly K. Schissel, Susana Wilson Hawken, Ann Boija, Charles Li, Isaac Klein, and Bradley L. Pentelute
Abstract
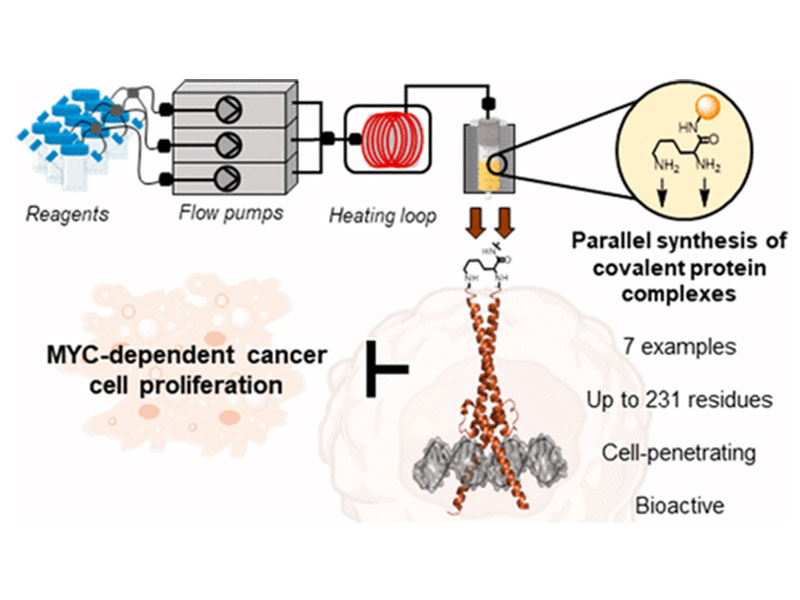
Dysregulation of the transcription factor MYC is involved in many human cancers. The dimeric transcription factor complexes of MYC/MAX and MAX/MAX activate or inhibit, respectively, gene transcription upon binding to the same enhancer box DNA. Targeting these complexes in cancer is a long-standing challenge. Inspired by the inhibitory activity of the MAX/MAX dimer, we engineered covalently linked, synthetic homo- and heterodimeric protein complexes to attenuate oncogenic MYC-driven transcription. We prepared the covalent protein complexes (∼20 kDa, 167–231 residues) in a single shot via parallel automated flow synthesis in hours. The stabilized covalent dimers display DNA binding activity, are intrinsically cell-penetrant, and inhibit cancer cell proliferation in different cell lines. RNA sequencing and gene set enrichment analysis in A549 cancer cells confirmed that the synthetic dimers interfere with MYC-driven transcription. Our results demonstrate the potential of automated flow technology to rapidly deliver engineered synthetic protein complex mimetics that can serve as a starting point in developing inhibitors of MYC-driven cancer cell growth.



