
A structural and mechanistic study of π-clamp-mediated cysteine perfluoroarylation
A structural and mechanistic study of π-clamp-mediated cysteine perfluoroarylation
Scientific Reportsvolume 7, Article number: 7954 (2017)
Published: 11 August 2017
Peng Dai, Jonathan K. Williams, Chi Zhang, Matthew Welborn, James J. Shepherd, Tianyu Zhu, Troy Van Voorhis, Mei Hong & Bradley L. Pentelute
Abstract
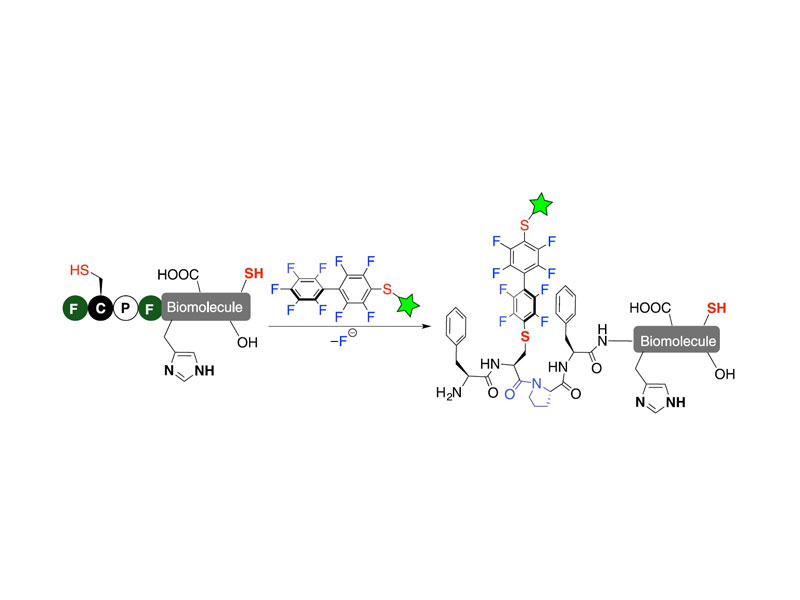
Natural enzymes use local environments to tune the reactivity of amino acid side chains. In searching for small peptides with similar properties, we discovered a four-residue π-clamp motif (Phe-Cys-Pro-Phe) for regio- and chemoselective arylation of cysteine in ribosomally produced proteins. Here we report mutational, computational, and structural findings directed toward elucidating the molecular factors that drive π-clamp-mediated arylation. We show the significance of a trans conformation prolyl amide bond for the π-clamp reactivity. The π-clamp cysteine arylation reaction enthalpy of activation (ΔH‡) is significantly lower than a non-π-clamp cysteine. Solid-state NMR chemical shifts indicate the prolyl amide bond in the π-clamp motif adopts a 1:1 ratio of the cis and trans conformation, while in the reaction product Pro3 was exclusively in trans. In two structural models of the perfluoroarylated product, distinct interactions at 4.7 Å between Phe1 side chain and perfluoroaryl electrophile moiety are observed. Further, solution 19F NMR and isothermal titration calorimetry measurements suggest interactions between hydrophobic side chains in a π-clamp mutant and the perfluoroaryl probe. These studies led us to design a π-clamp mutant with an 85-fold rate enhancement. These findings will guide us toward the discovery of small reactive peptides to facilitate abiotic chemistry in water.



